Science, Grade 9, Academic (SNC1D)
This course enables students to understand basic concepts in biology, chemistry, earth and space science, and physics; to develop skills in the processes of scientific inquiry; and to relate science to technology, society, and the environment. Students will learn scientific theories and conduct investigations related to cell division and reproduction; atomic and molecular structures and the properties of elements and compounds; the universe and space exploration; and the principles of electricity.
Biology: Reproduction
Overall Expectations
By the end of this course, students will:
- describe cell theory, and apply it to processes of cell division, including mitosis, and the function of sexual (including human) and asexual reproductive systems;
- investigate and analyse cell division and factors affecting cell reproduction;
- evaluate the implications for social decision making of scientific research and technological developments in reproductive biology.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- describe the major postulates of the cell theory and how the theory explains cell division (e.g., all living things are made up of one or more cells and the products of those cells; cells are the functional units of life; all cells come from pre-existing cells);
- describe cell division, including mitosis, as part of the cell cycle, including the roles of the nucleus, cell membrane, and organelles (e.g., stages of mitosis – prophase, metaphase, anaphase, and telophase);
- explain how the cell nucleus determines cellular processes and contains genetic material, and why DNA replication is important to organism survival;
- describe various types of asexual reproduction that occur in plant species or animal species, and various methods for the asexual propagation of plants (e.g., fission, budding, production of spores; fission in the amoeba and planaria flatworm, budding in the hydra and sponge; use of bulbs, cuttings, grafting, and modified stems in plants);
- describe and give examples of types of sexual reproduction that occur in plants and in animals, including hermaphrodites (e.g., conjugation, cross-fertilization, internal and external fertilization);
- compare sexual and asexual reproduction (e.g., asexual reproduction produces offspring whose DNA is identical to the parent’s DNA, given the same environment; sexual reproduction introduces variation to a species);
- describe the production, structure, and function of a mature egg and sperm in the development and formation of the zygote and embryo;
- describe, in general terms, the roles of hormones in human reproduction where there is no conception, and where conception, development, and parturition occur (e.g., the role that hormones produced in the pituitary gland play in regulating the development of ova or eggs);
- describe, in general terms, human development from conception to the growth of human organs and body proportions, including embryonic human development from early cleavage to the morphological stages;
- distinguish between somatic and reproductive cells and describe factors that may alter genetic material in both types of cells (e.g., uncontrolled exposure to a radioactive source and other mutagens).
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- formulate scientific questions related to reproduction (e.g., “What factors affect the health of the mother and foetus during the human pregnancy?”);
- demonstrate the skills required to plan and conduct an inquiry into reproduction, using instruments and tools safely, accurately, and effectively (e.g., use a microscope at an appropriate level of magnification to locate and view nuclear division on a slide);
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen (e.g., investigate the effects that ultraviolet radiation, carcinogens, water pollution, toxins, or nuclear radiation have on developing organisms);
- analyse qualitative and quantitative data and explain how the evidence gathered supports or refutes an initial hypothesis (e.g., propose an explanation for trends in the optimum reproductive years of women and, following data collection, evaluate the accuracy of that explanation);
- communicate scientific ideas, procedures, results, and conclusions using appropriate language and formats (e.g., describe the steps involved in spore and gamete production in mosses and explain the relationship between them);
- defend orally a given position on an issue or problem, based on their findings;
- use a microscope or microviewer to identify the various stages of mitosis (e.g., use prepared slides of mitosis);
- design and conduct an investigation into the stages of cell division to determine changes taking place in the nucleus and cell membrane (e.g., prepare slides of mitosis and observe them through a microscope);
- use a microscope to make scientific observations of an organism undergoing fission by the process of cell division (e.g., prepare a slide or use a prepared slide to draw an organism undergoing fission);
- predict the number of cell divisions required to produce a certain number of cells.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- demonstrate an understanding of the historical development of reproductive biology and outline the contribution of the microscope to knowledge in the field (e.g., describe the impact of the microscope on the development of scientific understanding of breeding);
- provide examples of how developments in reproductive biology have had an impact on global and local food production, populations, the spread of disease, and the environment (e.g., the impact of scientific developments in such areas as species preservation, genetic engineering of crops, or reproductive technologies);
- describe the importance of Canadian research and technological development in genetics and reproductive biology (e.g., describe and assess how techniques used to bring together nucleii of different plant species such as rye and wheat have improved hardiness and yield by producing the hybrid triticale);
- investigate careers that require an understanding of reproductive biology.
Chemistry: Atoms and Elements
Overall Expectations
By the end of this course, students will:
- describe various models of the atom, the atomic structure of common elements, and their organization in the periodic table;
- investigate the physical and chemical properties of elements and compounds and use the periodic table to predict the properties of elements;
- describe technologies associated with the refinement, use, and recycling of chemical elements and compounds.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- explain the characteristics and utility of a scientific model;
- describe and explain the particle theory of matter;
- describe an element as a pure substance made up of one type of particle or atom with its own distinct properties;
- recognize compounds as pure substances which may be broken down into elements by chemical means;
- demonstrate an understanding of compounds and elements by describing them in terms of molecules and atoms;
- describe the evolution of models of the atom (e.g., from Dalton to Bohr);
- describe the Bohr-Rutherford model of atomic structure and apply it to atoms and their common ions to atomic number 20;
- identify general features of the periodic table (e.g., arrangement of the elements based on atomic structure, groups or families of elements, periods or horizontal rows);
- relate the Bohr-Rutherford atomic model to properties of elements and their positions in the periodic table;
- compare similarities in properties both between and within families of elements to similarities in their atomic structure (e.g., alkali metals, halogens, noble gases);
- use the periodic table to predict the physical and chemical characteristics of an element (e.g., predict that a metal such as sodium will be extremely reactive with a non-metal such as chlorine);
- identify and write the symbols for common elements and the
formulae for common compounds (e.g., C, Cl, S, N; H
2 O, CO2 , NaCl); - solve density problems – given any two of mass, volume, and
density, determine the third – using the formula
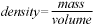 and appropriate SI units;
and appropriate SI units;
- describe, through observations, the evidence for chemical changes (e.g., changes in colour, production of a gas, formation of a precipitate, production or absorption of heat, production of light);
- identify, through their observations, the characteristic physical and chemical properties of common elements and compounds (e.g., aluminum is a good conductor of heat; magnesium reacts with oxygen to produce magnesium oxide).
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- demonstrate knowledge of laboratory, safety, and disposal procedures while conducting investigations (e.g., wear safety glasses; practise orderliness and cleanliness; be aware of WHMIS guidelines and emergency procedures; be aware of proper handling and storage procedures);
- formulate scientific questions about physical and chemical properties of elements and compounds;
- demonstrate the skills required to plan and conduct an inquiry into the properties of elements and compounds, using instruments, tools, and apparatus safely, accurately, and effectively (e.g., investigate the reactions of some metals and some non-metals);
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- gather and record qualitative and quantitative data using an appropriate format, and analyse the data to explain how the evidence gathered supports or refutes an initial hypothesis (e.g., conclude from data obtained from the electrolysis of water that the proportion of hydrogen to oxygen in water molecules is 2:1);
- communicate scientific ideas, procedures, results, and conclusions using appropriate SI units, language, and formats, and evaluate the processes used in planning, problem solving, decision making, and completing the task (e.g., use appropriate vocabulary such as substance, compound, element, atomic number, mass number);
- formulate definitions of major variables and other aspects of their investigations (e.g., define mass, electrons, protons, neutrons, ions, and isotopes);
- design and conduct experiments to determine the physical and chemical properties of everyday and common laboratory substances such as carbon, copper nitrate, starch, and wax (e.g., physical properties: colour, change of state, solubility; chemical properties: combustibility, reaction with water);
- use molecular models to illustrate the structure of simple
molecules (e.g., H
2 , O2 , H2 O, NH3 , CH4 , CO2 ); - use proper notation to represent elements, including their
atomic number and mass number (e.g., represent the C-12 isotope,
which has an atomic number of 6 and a mass number of 12, as
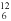 C).
C).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- describe the methods used to extract elements in Canada, and outline associated economic and environmental considerations (e.g., use various sources to explain how gold, nickel, carbon, or uranium is obtained and refined);
- compare the physical and chemical properties of elements to assess their potential uses and associated risks (e.g., hydrogen versus helium in balloons, copper versus aluminum in wiring, copper versus lead in plumbing);
- describe technologies that have depended on understanding atomic and molecular structure (e.g., television, X-rays, nuclear medicine, nuclear power, electron microscopy);
- investigate potential careers associated with an understanding of the physical and chemical properties of elements and compounds.
Earth and Space Science: The Study of the Universe
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of how scientific evidence and technological advances support the development of theories about the formation, evolution, structure, and nature of our solar system and the universe;
- investigate and predict the appearance and motion of visible celestial objects;
- evaluate how human endeavours and interest in space have contributed to our understanding of outer space, the Earth, and living things, and describe Canadian contributions to space exploration.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- describe and compare the major components of the universe, using appropriate scientific terminology and units (e.g., record the location and movement of planets and satellites, and of stars, galaxies, and clusters of galaxies, using Astronomical Units and light years);
- describe the generally accepted theory of the origin and evolution of the universe (i.e., the “big bang” theory) and the observational evidence that supports it;
- describe and compare the general properties and motions of the components of the solar system (e.g., the composition and the physical properties – such as size and state, rotation, size and period of orbit – of the Sun, planets, moons, asteroids, comets);
- describe and explain the effects of the space environment on organisms and materials (e.g., the effects of microgravity on organisms in a spacecraft);
- outline the generally accepted theory of the formation of the solar system (i.e., that it was formed from a contracting, spinning disc of dust and gas);
- describe the Sun and its effects on the Earth and its atmosphere (e.g., explain the importance of the Sun as an energy source and the types of radiation emitted; describe the aurora borealis);
- outline models and theories for describing the nature of the Sun and stars and their origin, evolution, and fate.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- formulate scientific questions about the motion of visible celestial objects;
- plan ways to model and/or simulate an answer to the questions chosen (e.g., determine, using scale models, and describe, using appropriate astronomical units, how astronomers are able to understand and compare the sizes and distances of objects in the solar system, and in the universe beyond);
- demonstrate the skills required to plan and conduct an inquiry into the motion and characteristics of visible celestial objects, using instruments, tools, and apparatus safely, accurately, and effectively;
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen (e.g., analyse and predict the time required for a spacecraft to travel to the Moon, or to another planet or moon in the solar system, and investigate the factors which limit the feasibility of the voyage – such as fuel, costs, time, comfort, safety, speed of travel, and human requirements);
- gather, organize, and record information using a format that is appropriate to the investigation (e.g., maintain a log of observations of changes in the night sky; prepare a comparative data table on various stars);
- analyse qualitative and quantitative data, and explain how evidence gathered supports or refutes an initial hypothesis (e.g., determine the actual size of a celestial object from its distance and its apparent size);
- communicate scientific ideas, procedures, results, and conclusions using appropriate SI units, language, and formats;
- calculate and compare the sizes of, and the distances to, objects in the solar sytem and in the universe beyond, using appropriate SI units;
- predict the qualitative and quantitative characteristics of visible celestial objects (e.g., determine the temperature of a star by observing its colour; predict the next appearance of a comet from the time of its last appearance and the period of its orbit).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- describe, evaluate, and communicate the impact of research and other accomplishments in space technology on our understanding of scientific theories and principles and on other fields of endeavour (e.g., advances in fluid physics, crystal growth, and material science, and in technologies associated with robotics, agriculture, and telecommunications);
- investigate the ways in which Canada participates in space research and international space programs (e.g., the International Space Station, telecommunications, satellite technology);
- describe and explain how data provided by ground-based astronomy, satellite-based astronomy, and satellite exploration of the Sun, planets, moons, and other solar-system objects contribute to our knowledge of the solar system;
- explore science and technology careers that are related to the exploration of space, and identify their educational requirements.
Physics: The Characteristics of Electricity
Overall Expectations
By the end of this course, students will:
- describe and apply models of static and current electricity;
- design and conduct investigations into electrical circuits found in everyday life and into the quantitative relationships among current, potential difference, and resistance;
- evaluate the social, economic, and environmental costs and benefits arising from the methods of electrical energy production used in Canada.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- describe the properties of static electric charges, and explain electrostatic attraction and repulsion using scientific models of atomic structure;
- describe charging by contact and by induction;
- compare qualitatively static electricity and electric current (e.g., the charge on a charged electroscope and the charge in an operating circuit);
- describe the concepts of electric current, potential difference, and resistance, with the help of a water analogy;
- explain how electric current, potential difference, and electrical resistance are measured using an ammeter and a voltmeter;
- state the SI units of potential difference, electric current, electrical resistance, electrical energy, and power (e.g., volt, ampere, ohm, joule, watt, and kilowatt);
- describe the relationship among electrical resistance R, potential difference V, and current I;
- solve simple problems involving these quantities (V=IR);
- describe the potential difference and current characteristics in a series and a parallel circuit;
- compare the electrical resistance of a series and a parallel connection of identical resistors to that of a single resistor;
- determine quantitatively the percent efficiency of an electrical
device that converts electrical energy to other forms of energy,
using the relationship
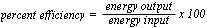
- describe the relationship among electrical energy transformed E,
power P, and elapsed time
 t, and solve
simple problems involving these physical quantities (E=P
t, and solve
simple problems involving these physical quantities (E=P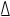 t);
t);
- compare methods of producing electrical energy, including their advantages and disadvantages (e.g., voltaic cells; primary and secondary cells; photoelectric cells and thermocouples; hydro-electric and fossil-fuelled power; wind, and tidal power).
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- demonstrate knowledge of electrical safety procedures when planning and carrying out an inquiry and choosing and using materials, tools, and equipment;
- formulate scientific questions about electricity and restate them in a testable form, identifying the relationships among variables (e.g., “What is the relationship among the number of dry cells connected, in series or in parallel, the potential difference of the source, and the electric current that passes through a resistor?”);
- demonstrate the skills required to plan and conduct an inquiry into electricity, using instruments, tools, and apparatus safely, accurately, and effectively (e.g., use an ammeter and a voltmeter to measure current and potential difference in a circuit);
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- gather and record qualitative and quantitative data using an appropriate format, and analyse the data to explain how the evidence gathered supports or refutes an initial hypothesis (e.g., explain the variations in the monthly costs of electrical energy);
- communicate ideas, procedures, results, and conclusions using appropriate SI units, language, and formats, and evaluate the processes used in planning, problem solving, decision making, and completing the task;
- design, draw, and construct series and parallel circuits for a given purpose, and measure current, potential difference, and resistance at various points in the circuit, using appropriate instruments and SI units (e.g., design and construct a circuit used to enable one of several light bulbs to be switched on and off independently of the others);
- formulate operational definitions for physical quantities involved in electricity (e.g., potential difference, current, resistance, electrical energy, and power);
- charge an electroscope by contact and by induction;
- predict, verify, and explain the effect of a nearby charged object on a charged electroscope;
- use appropriate instruments and techniques to investigate potential difference against current for an ohmic resistor in a simple series circuit, graph the data, and determine resistance from the slope of the graph.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- explain practical applications of static and current electricity (e.g., an air cleaner, an electrostatic paint sprayer);
- devise a plan for a self-contained system to generate energy, using renewable energy sources, to meet the energy requirements of a dwelling, farm, or community in Ontario (e.g., design a plan to use any combination of wind, solar, or hydroelectric power);
- identify problems related to electrostatic charge in everyday situations and evaluate solutions (e.g., use of static straps to reduce charge build-up in automobiles; use of electrostatic precipitators to decrease pollution; use of lightning rods to protect buildings).
Science, Grade 9, Applied (SNC1P)
This course enables students to understand basic concepts in biology, chemistry, earth and space science, and physics; to develop practical skills in scientific investigation; and to apply their knowledge of science to everyday situations. Students will design and conduct investigations into practical problems and issues related to cell division and reproduction, the structure and properties of elements and compounds, astronomy and space exploration, and static and current electricity.
Biology: Reproduction – Processes and Applications
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of the processes of cell division, including mitosis, and the function of sexual (including human) and asexual reproductive systems;
- conduct investigations into questions arising from reproductive issues;
- examine the impact of scientific research and technological developments on issues related to reproduction.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- describe the basic process of cell division, including what happens to the cell membrane and the contents of the nucleus (e.g., stages of mitosis – prophase, metaphase, anaphase, and telophase);
- demonstrate an understanding of the importance of cell division to the growth and reproduction of an organism (e.g., describe changes in cell division in an organism during its lifespan);
- demonstrate an understanding that the nucleus of a cell contains genetic information and determines cellular processes;
- describe various types of asexual reproduction that occur in plant species or in animal species and various methods for the asexual propagation of plants (e.g., fission, budding, production of spores; fission in the amoeba and planaria flatworm, budding in the hydra and sponge; use of bulbs, cuttings, grafting, and modified stems in plants);
- describe the various types of sexual reproduction that occur in plants and in animals, and identify some plants and animals, including hermaphrodites, that exhibit this type of reproduction (e.g., conjugation, cross-fertilization, internal and external fertilization);
- compare sexual and asexual reproduction (e.g., asexual reproduction does not require a partner and can take place whenever environmental conditions such as food, warmth, and moisture are suitable);
- explain signs of pregnancy in humans and describe the major stages of human development from conception to early infancy.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- identify a current problem or concern relating to plant or animal reproduction (e.g., development of hybrid species);
- formulate scientific questions about the problem or concern, and develop a plan to answer these questions;
- demonstrate the skills required to plan and conduct an inquiry into reproduction, using instruments and tools safely, accurately, and effectively (e.g., use a microscope at an appropriate level of magnification to locate and view mitosis on a slide);
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- organize, record, and analyse the information gathered (e.g., interpret patterns and trends; discuss relationships among variables; and predict consequences of action or inaction);
- predict the value of a variable by interpolating or extrapolating from graphical data (e.g., graph data on the optimum reproductive years of women and predict trends for upcoming years);
- communicate scientific ideas, procedures, results, and conclusions using appropriate language and formats;
- defend orally a position on the concern or problem investigated;
- use a microscope to observe and identify (in living tissue and prepared slides) animal and vegetable cells in different stages of mitosis, as well as cells undergoing asexual reproduction (e.g., budding in yeast).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- describe the use of reproductive technologies in a workplace environment and explain the costs and benefits of using such technologies (e.g., use of reproductive technologies by: a horticulturalist – cloning; a doctor – in vitro fertilization; a farmer or breeder – selective breeding processes);
- examine some Canadian contributions to research and technological development in the field of genetics and reproductive biology (e.g., describe the development of the McIntosh apple or of canola; do research on foetal alcohol syndrome or cystic fibrosis);
- identify local environmental factors and individual choices that may lead to a change in a cell’s genetic information or an organism’s development, and investigate the consequences such factors and choices have on human development (e.g., identify the consequences of exposure to X-rays or the use of cigarettes or illegal drugs for the development of the foetus);
- provide examples of the impact of developments in reproductive biology on global and local food production, populations, the spread of disease, and the environment (e.g., genetic engineering of crops; reproductive technologies and the production of hybrid species);
- describe careers that involve some aspect of reproductive biology.
Chemistry: Exploring Matter
Overall Expectations
By the end of this course, students will:
- describe the atomic structure of common elements and their organization in the periodic table;
- investigate the physical and chemical properties of common elements and compounds, and relate the properties of elements to their location in the periodic table;
- demonstrate an understanding of the importance, production, use, and environmental hazards of common elements and simple compounds.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- describe an element as a pure substance made up of one type of particle or atom with its own distinct properties;
- recognize compounds as pure substances that may be broken down into elements by chemical means;
- describe compounds and elements in terms of molecules and atoms;
- identify each of the three fundamental particles (neutron, proton, and electron), and its charge, location, and relative mass in a simple atomic model (e.g., the Bohr-Rutherford model);
- identify general features of the periodic table (e.g., arrangement of the elements based on atomic structure, groups or families of elements, periods or horizontal rows);
- demonstrate an understanding of the relationship between the properties of elements and their position in the periodic table (e.g., metals appear on the left of the periodic table; non-metals appear on the right);
- identify and write symbols/formulae for common elements and
compounds (e.g., H, Mg, S, N and NaCl, O
2 , H2 O, CO2 ); - describe, using their observations, the evidence for chemical changes (e.g., energy change, formation of a gas or precipitate, change in colour or odour, change in temperature);
- distinguish between metals and non- metals and identify their characteristic properties (e.g., most metals are lustrous or shiny and good conductors of heat; most non-metals in solid form are brittle and not good conductors of heat).
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- demonstrate knowledge of laboratory, safety, and disposal procedures while conducting investigations (e.g., wear safety glasses; practise orderliness and cleanliness; follow WHMIS guidelines and emergency procedures; use proper procedures for handling and storage);
- determine how the properties of substances influence their use (e.g., how the reactions of metals with air influence their use);
- formulate scientific questions about a problem or issue involving the properties of substances;
- demonstrate the skills required to plan and conduct an inquiry into the properties of substances, using apparatus and materials safely, accurately, and effectively (e.g., investigate the physical properties of common elements and classify them as metals or non-metals);
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- organize, record, and analyse the information gathered (e.g., interpret patterns and trends; discuss relationships among variables; predict consequences of action or inaction);
- communicate scientific ideas, procedures, results, and conclusions using appropriate language and formats (e.g., present data on different chemical substances in a table using appropriate headings such as compound, element, chemical property, physical property);
- investigate, by laboratory experiment or classroom demonstration, the chemical properties of representative families of elements (e.g., combustibility, reaction with water of Mg, Ca or C, Si);
- investigate the properties of changes in substances, and classify them as physical or chemical based on experiments (e.g., solubility, combustibility, change of state, changes in colour);
- construct molecular models of simple molecules (e.g., H
2 , O2 , H2 O, NH3 , CH4 , CO2 ).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- identify uses of elements in everyday life (e.g., iron and other elements in steel; aluminum, oxygen, chlorine in water);
- describe the methods used to obtain elements in Canada, and outline local environmental concerns and health and safety issues related to the ways in which they are mined and processed (e.g., explain how gold, nickel, carbon, or uranium is obtained and processed);
- explain how a knowledge of the physical and chemical properties of elements enables people to determine the potential uses of the elements and assess the associated risks (e.g., helium versus hydrogen in balloons, copper versus aluminum in wiring, copper versus lead in plumbing);
- identify and describe careers that require knowledge of the physical and chemical properties of elements and compounds.
Earth and Space Science: Space Exploration
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of the formation, evolution, structure, and nature of our solar system and of the universe;
- design and conduct investigations into the appearance and motion of visible celestial objects;
- describe how human endeavours and interest in space have contributed to our understanding of outer space, the Earth, and living things, and identify Canadian contributions to space exploration.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- recognize and describe the major components of the universe using appropriate scientific terminology and units (e.g., record the location and movement of planets and satellites, stars, galaxies, and clusters of galaxies using Astronomical Units and light years);
- describe the generally accepted theory of the origin and evolution of the universe (i.e., the “big bang” theory) and the observational evidence that supports it;
- describe, compare, and contrast the general properties and motions of the components of the solar system (e.g., the composition and physical properties – such as size and state, rotation, size and period of orbit – of the Sun, planets, moons, asteroids, comets);
- describe the Sun and its effects on the Earth and its atmosphere (e.g., the Sun as an energy source, solar activity, aurora borealis);
- describe and explain the effects of the space environment on organisms and materials (e.g., the effects of microgravity and temperature on organisms during space exploration).
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- identify problems and issues that scientists face when investigating celestial objects and describe ways these problems can be solved (e.g., use of a telescope to collect light from a faint object);
- formulate scientific questions about a problem or issue in space exploration;
- demonstrate the skills required to plan and conduct an inquiry about space exploration, using instruments, tools, and apparatus safely, accurately, and effectively;
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- organize, record, and analyse the information gathered (e.g., interpret patterns and trends; discuss relationships among variables; predict consequences of action or inaction);
- communicate scientific ideas, procedures, results, and conclusions using appropriate SI units, language, and formats (e.g., prepare a comparative data table on various stars);
- conduct investigations on the motion of visible celestial objects, using instruments, tools, and apparatus safely, accurately, and effectively (e.g., graph sunrise and sunset data and relate them to the motions of the Earth);
- gather, organize, and record data through regular observations of the night sky and/or use of appropriate software programs, and use these data to identify and study the motion of visible celestial objects (e.g., track the position of the Moon and planets over time).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- identify and assess the impact of developments in space research and technology on other fields of endeavour (e.g., the advancement of robotics, agriculture, resource management, navigation, and telecommunications);
- relate the beliefs of various cultures concerning celestial objects to aspects of their civilization (e.g., aboriginal beliefs, Greek mythology, Mayan civilization);
- provide examples of the contributions of Canadian research and development to space exploration and technology;
- explore careers in science and technology that are related to the exploration of space, and identify their educational requirements.
Physics: Electrical Applications
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of the principles of static and current electricity;
- design and build electrical circuits that perform a specific function;
- analyse the practical uses of electricity and its impact on everyday life.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- explain common electrostatic phenomena (e.g., clothes that “stick” together, attraction of hairs to combs);
- compare qualitatively static and current electricity (e.g., a charge on a charged electroscope and the charge in an operating circuit);
- describe the concepts of electric current, potential difference, and resistance, with the help of a water analogy;
- explain how electric current, potential difference, and resistance are measured using an ammeter and a voltmeter;
- describe qualitatively the effects of varying electrical resistance and potential difference on electric current in an electrical circuit;
- apply the relationship potential difference=resistance x current to simple series circuits;
- determine quantitatively the percent efficiency of an electrical
device that converts electrical energy to other forms of energy,
using the relationship
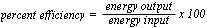
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- demonstrate knowledge of electrical safety procedures when planning and carrying out investigations and choosing and using materials, tools, and equipment;
- identify an authentic practical challenge or problem related to the use of electricity (e.g., to design household wiring; to increase the efficiency of electrical usage in the school);
- formulate questions about the problem or issue;
- demonstrate the skills required to plan and conduct an inquiry into the use of electricity, using instruments, tools, and apparatus safely, accurately, and effectively;
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- organize, record, and analyse the information gathered (e.g., interpret patterns and trends; discuss relationships among variables; and predict consequences of action or inaction);
- communicate scientific ideas, procedures, results, and conclusions using appropriate SI units, language, and formats (e.g., electrical power, voltage, resistance; drawings, charts, graphs);
- design, draw, and construct series and parallel circuits that perform a specific function (e.g., given light bulbs, wires, and batteries, produce circuits with: one light bulb on; two light bulbs of the same brightness; one light bulb disconnected and the other light bulb on);
- use appropriate instruments to collect and graph data, and determine the relationship between voltage and current in a simple series circuit with a single resistor.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- describe and explain household wiring and its typical components (e.g., parallel circuits with switches, fuses, circuit breakers, outlets);
- develop a solution to a practical problem related to the use of electricity in the home, school, or community (e.g., choose an appropriate fuse or circuit breaker for a specific circuit);
- compare electrical energy production technologies, including risks and benefits (e.g., explain the advantages and disadvantages of using hydro, photovoltaic, wind, and tidal generators to produce electrical energy);
- explain how some common household electrical appliances operate (e.g., electric kettle, electric baseboard heater, electric light bulb);
- describe careers that involve electrical technologies, and use employability- assessment programs, newspaper job advertisements, and/or appropriate Internet sources to identify the knowledge and skill requirements of such careers.
Science, Grade 10, Academic (SNC2D)
This course enables students to develop a deeper understanding of concepts in biology, chemistry, earth and space science, and physics; to develop further their skills in scientific inquiry; and to understand the interrelationships among science, technology, and the environment. Students will conduct investigations and understand scientific theories related to: ecology and the maintenance of ecosystems; chemical reactions, with particular attention to acid-base reactions; factors that influence weather systems; and motion.
Biology: The Sustainability of Ecosystems
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of the dynamic nature of ecosystems, including the relationship between ecological balance and the sustainability of life;
- investigate factors that affect ecological systems and the consequences of changes in these factors;
- analyse issues related to environmental sustainability and the impact of technology on ecosystems.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- describe the processes of photosynthesis and cellular respiration as they relate to the cycling of energy, carbon, and oxygen through abiotic and biotic components of an ecosystem (e.g., explain that photosynthesis and cellular respiration are essentially reverse processes, and identify the reactants and products of their overall reactions);
- illustrate the cycling of matter through biotic and abiotic components of an ecosystem by tracking nitrogen;
- explain the process of bioaccumulation and assess its potential impact on the viability and diversity of consumers at all trophic levels;
- examine the factors (natural and external) that affect the survival and equilibrium of populations in an ecosystem (e.g., resource limits of an ecosystem, competing populations, bioaccumulation, selective decline);
- examine how abiotic factors affect the survival and geographical location of biotic communities (e.g., explain why deserts exist in different parts of the world);
- explain why different ecosystems respond differently to short-term stresses and long-term changes (e.g., short term: the activity of tent caterpillars during a season; long-term: the effect of acid rain on maple trees);
- compare a natural and a disturbed ecosystem and suggest ways of assuring their sustainability (e.g., compare a meadow and a lawn);
- explain how soil composition and fertility can be altered in an ecosystem and identify the possible consequences of such changes.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- formulate scientific questions about observed ecological relationships, ideas, problems, and issues (e.g., “What impact will supplying an excess of food for a particular organism have on an ecosystem?”);
- demonstrate the skills required to plan and conduct an inquiry into ecological relationships, using instruments, apparatus, and materials safely and accurately, and controlling major variables and adapting or extending procedures where required;
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- analyse data and information and evaluate evidence and sources of information, identifying flaws such as errors and bias;
- select and use appropriate vocabulary and numeric, symbolic, graphic, and linguistic modes of representation to communicate scientific ideas, plans, results, and conclusions (e.g., use terms such as biotic, abiotic, biomass, biome, ecosystem, chemical concentration, and biodiversity when making presentations);
- design and conduct an investigation to examine the effects of one factor on soil composition and fertility and on water quality in an ecosystem (e.g., design and conduct an experiment to examine the effects of altering soil pH on the fertility of plants and on the concentration of dissolved oxygen in water, and graph the results);
- analyse a population case study (e.g., of deer, wolves, or humans) by producing population growth curves for each of the populations in the study, and use the graphs to explain how different factors affect population size and to predict the effect of varying factors (e.g., the availability of food) on the population.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- assess the impact of technological change and natural change on an ecosystem (e.g., the introduction of fertilizer and pesticides to soil; the introduction of a genetically engineered plant or the effect of polluted water or air on plants and animals; the effect on an ecosystem of forest fire, flood, the natural infection of one species, or the movement of a species in or out of the area);
- describe ways in which the relationships between living organisms and their ecosystems are viewed by other cultures (e.g., First Nations);
- identify and research a local issue involving an ecosystem; propose a course of action, taking into account human and environmental needs; and defend their position in oral or written form (e.g., organize and participate in a debate on converting a grass lot into a parking lot);
- describe the physical and chemical processes involved in the methods used to clean up a contaminated site (e.g., how absorbent chemicals such as charcoal work in cleaning up oil spills);
- identify and evaluate Canadian initiatives in protecting Canada’s ecosystems;
- explain changes in popular views about the sustainability of ecosystems and humans’ responsibility in preserving them (e.g., the shift from a belief that all resources are inexhaustible to the belief that recycling, reusing, and reducing are important);
- describe careers that involve knowledge of ecology or environmental technologies, and use resources such as the Internet to determine the knowledge and skill requirements of such careers.
Chemistry: Chemical Processes
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of chemical reactions, the symbolic systems used to describe them, and the factors affecting their rates;
- design and conduct investigations of chemical reactions, using standard scientific procedures, and communicate the results;
- determine why knowledge of chemical reactions is important in developing consumer products and industrial processes and in addressing environmental concerns.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- recognize the relationships among chemical formulae, composition, and names;
- explain, using the law of conservation of mass and atomic theory, the rationale for balancing equations;
- describe, using their observations, the reactants and products of a variety of chemical reactions, including synthesis, decomposition, and displacement reactions (e.g., the burning of magnesium, the production of oxygen from hydrogen peroxide, the reaction of iron in copper sulphate);
- describe and explain qualitatively how factors such as energy, concentration, and surface area can affect rates of chemical reactions;
- explain the interrelationships among metals and non-metals, acidic and basic oxides, and acids, bases, and salts;
- describe qualitatively acid-base neutralization through observation of simple acid-base reactions;
- describe how the pH scale is used to identify the acidity of solutions;
- name and write the formulae of common ionic and molecular
compounds (e.g., H
2 SO4 , NaNO3 , CO2 , NaOH), using a periodic table and an IUPAC table of ions.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- select and use appropriate apparatus, and apply WHMIS safety procedures for the handling, storage, disposal, and recycling of laboratory materials (e.g., wear safety goggles and aprons; use proper techniques for the handling, disposal, and recycling of acids, bases, and heavy metal ions; describe procedures to be followed in an emergency);
- formulate scientific questions about practical problems and issues involving chemical processes (e.g., “How does varying the concentration of a reactant affect the rate of a reaction?”);
- demonstrate the skills required to plan and conduct an inquiry into chemical processes using a broad range of tools and techniques safely and accurately, and controlling major variables and adapting or extending procedures where required (e.g., neutralize a dilute solution of sodium hydroxide with dilute hydrochloric acid and isolate the sodium chloride produced);
- select and integrate information from various sources, including electronic and print resources, community resources, and personally collected data, to answer the questions chosen;
- analyse data and information and evaluate evidence and sources of information, identifying flaws such as errors and bias;
- describe experimental procedures in the form of a laboratory report (e.g., clearly identify the variable under investigation as well as the variables controlled; clearly describe the procedures followed and the data obtained; write an analysis of what was learned from the data);
- select and use appropriate vocabulary, SI units, and numeric, symbolic, graphic, and linguistic modes of representation to communicate scientific ideas, plans, results, and conclusions (e.g., descriptions of experimental procedures using the scientific method; data presented in tables);
- represent simple chemical reactions using molecular models, word equations, and balanced chemical equations;
- compare theoretical and empirical values and account for discrepancies when investigating conservation of mass (e.g., measure the mass of a chemical reaction system – such as the reaction of iron (III) nitrate and dilute sodium hydroxide – before and after a change, and account for any discrepancies);
- conduct experiments to identify the acidity and basicity of some common substances (e.g., use acid-base indicators to classify common household substances according to the pH scale);
- conduct experiments on the combustion of metals and non-metals and react the oxides formed with water to produce acidic or basic solutions;
- design an experiment to determine qualitatively the factors that influence chemical reactions (e.g., an experiment to measure the effect of surface area on rate of reaction);
- conduct appropriate chemical tests to identify common gases (e.g., oxygen, hydrogen, carbon dioxide).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- explain how environmental challenges can be addressed through an understanding of chemical substances (e.g. challenges such as the renewal of the Great Lakes, the neutralization of acid spills, the scrubbing of waste gases in smokestacks);
- describe how an understanding of chemical reactions has led to the development of new consumer products and technological processes (e.g., antacids, fire-retardant materials);
- identify everyday examples where the rates of chemical reactions are modified (e.g., the use of kindling to increase surface area in order to start a fire; the refrigeration of food to slow down spoilage);
- describe careers based on technologies that utilize chemical reactions.
Earth and Space Science: Weather Dynamics
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of the factors affecting the fundamental processes of weather systems;
- investigate and analyse trends in local and global weather conditions to forecast local and global weather patterns;
- evaluate how technology has contributed to our understanding of the physical factors that affect the weather.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- identify and describe the principal characteristics of the hydrosphere and the four regions of the atmosphere;
- describe and explain heat transfer within the water cycle and how the hydrosphere and atmosphere act as heat sinks;
- describe and explain heat transfer in the hydrosphere and atmosphere and its effects on air and water currents;
- describe and explain the effects of heat transfer within the hydrosphere and atmosphere on the development, severity, and movement of weather systems (e.g., effects such as pressure gradients, cloud formation, winds);
- explain different types of transformations of water vapour in the atmosphere and their effects (e.g., clouds, hail, freezing rain, ice pellets, fog, frost, rain, snow);
- describe the factors contributing to earth temperature gradients and to wind speed and direction;
- describe cyclones, hurricanes, tornadoes, and monsoons in terms of the meeting of air masses, atmospheric humidity, and the jet stream.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- formulate scientific questions about weather-related phenomena, problems, and issues (e.g., “What is the effect of heat energy transfer within the hydrosphere?”);
- demonstrate the skills required to plan and conduct a weather-related inquiry, using a broad range of tools and techniques safely and accurately, and adapting or extending procedures where required (e.g., determine how the accuracy of weather predictions can be maintained when data from several places and people are combined);
- select and integrate information from various sources, including electronic and print resources, to answer the questions chosen;
- analyse data and information and evaluate evidence and sources of information, identifying flaws such as errors and bias (e.g., explain possible sources of error when interpreting a satellite picture used for predicting weather);
- select and use appropriate vocabulary and numeric, symbolic, graphic, and linguistic modes of representation to communicate scientific ideas, plans, results, and conclusions (e.g., use historical and current weather data to support a position on future weather patterns);
- investigate factors which affect the development, severity, and movement of global and local weather systems (e.g., the ozone layer, El Niño, bodies of water, glaciers, smog, rain forests).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- explain the role of weather dynamics in environmental phenomena and consider the consequences to humans of changes in weather (e.g., the role of weather in air pollution, acid rain, global warming, and smog; the fact that smog aggravates asthma);
- explain how people have utilized their understanding of weather patterns for various purposes (e.g., to harness wind as a power source; to participate in ocean sailing races);
- compare various cultural (e.g., First Nations) and historical views on the origins and interpretations of weather;
- explain how a scientific understanding of weather patterns can be used to modify environmental conditions (e.g., by seeding clouds to alleviate drought; by modelling the dynamics of fire-fighting strategies to fight forest fires);
- describe examples of technologies, particularly those of Canadian origin, that contribute to the field of meteorology (e.g., satellite imaging).
Physics: Motion
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of different kinds of motion and of the quantitative relationships among displacement, velocity, and acceleration, and solve simple problems involving displacement, velocity, and acceleration;
- design and conduct investigations on the displacement, velocity, and acceleration of an object;
- analyse everyday phenomena and technologies in terms of the motions involved.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- distinguish among and provide examples of scalar and vector
quantities as they relate to the description of linear motion (e.g.,
among distance
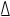 d,
displacement
d,
displacement
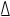
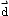 ,
and position
,
and position
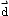 ,
and between speed v and velocity
,
and between speed v and velocity
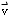 );
);
- add collinear displacement vectors algebraically and graphically and non-collinear displacement vectors graphically;
- distinguish among constant, instantaneous, and average speed and among constant, instantaneous, and average velocity, and give examples involving uniform and non-uniform motion;
- describe quantitatively the relationship among one-dimensional
average speed v
av , distance travelled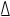 d,
and elapsed time
d,
and elapsed time
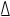 t,
and solve simple problems involving these physical quantities
t,
and solve simple problems involving these physical quantities
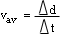 ;
;
- describe quantitatively the relationship among one-dimensional
average velocity
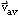 ,
displacement
,
displacement
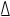
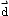 ,
and elapsed time
,
and elapsed time
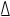 t,
and solve simple problems involving these physical quantities
t,
and solve simple problems involving these physical quantities
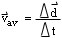
- draw position-time graphs and calculate the average velocity and instantaneous velocity from such graphs;
- describe quantitatively the relationship among one-dimensional
average acceleration
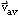 ,
change in velocity
,
change in velocity
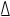
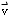 ,
and elapsed time
,
and elapsed time
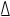 t,
and solve simple problems involving these physical quantities
t,
and solve simple problems involving these physical quantities
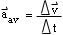
- draw position-time and velocity-time graphs for constant velocity and for constant acceleration, and calculate the constant acceleration and displacement from velocity-time graphs;
- use a velocity-time graph for constant acceleration to derive
the equation for average velocity
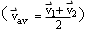
and the equations for displacement
[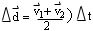 and
and
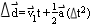 ]
]
and solve simple problems in one dimension using these equations.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- formulate scientific questions about observed relationships, ideas, problems, and issues related to motion (e.g., “What are the different acceleration characteristics of different transportation vehicles?”);
- demonstrate the skills required to plan and conduct an inquiry into motion, controlling major variables and adapting or extending procedures where required (e.g., determine the time or distance intervals at which measurements should be taken to calculate the average velocity of a bicycle rider);
- use a broad range of tools and techniques safely, accurately, and effectively to compile, record, and analyse data and information, and apply mathematical and conceptual models to develop and assess possible explanations (e.g., stopwatches, photo-gates, length-measurement devices, and motion sensors to obtain data; electronic spreadsheets and graphs to record and analyse the data);
- select and integrate information from various sources, including electronic and print resources, to answer the questions chosen;
- analyse data and information and evaluate evidence and sources of information, identifying flaws such as errors and bias (e.g., determine the mathematical relationship among displacement, velocity, and time, and identify any sources of error in data collection);
- identify, explain, and express sources of error and uncertainty in experimental measurements;
- select and use appropriate vocabulary, SI units, and numeric, symbolic, graphic, and linguistic modes of representation to communicate scientific ideas, plans, results, and conclusions (e.g., present a graph showing an object’s velocity, ensuring that the variables are on the appropriate axis);
- design, conduct, and evaluate experiments to measure the displacement, velocity, and acceleration of a moving object in one dimension, for both uniform motion and constant acceleration;
- design, conduct, and evaluate an experiment to measure acceleration due to gravity;
- use simple graphs and vector diagrams to describe predicted and observed motion in one dimension.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- evaluate the costs and benefits, including the safety and environmental factors, of technologies which have enabled us to travel at ever-greater speeds, and the impact of the increased capacity for speed on risk behaviour and subsequent injuries (e.g., snowmobiles, automobiles, motorized personal water craft);
- describe the development of those features of a piece of sports equipment which relate to improving performance (e.g., a baseball, skates, a skateboard, in-line skates, a snowboard, a bicycle);
- analyse how technology is used for tracking the motion of objects and outline the kinds of scientific knowledge gained through the use of such technologies (e.g., the tracking of animal migrations, airplane flights, traffic, ocean currents).
Science, Grade 10, Applied (SNC2P)
This course enables students to develop a deeper understanding of concepts in biology, chemistry, earth and space science, and physics; to develop further their practical skills in scientific investigation; and to apply their knowledge of science to real-world situations. Students will design and conduct investigations into everyday problems and issues related to ecological sustainability, chemical reactions, weather systems, and motion.
Biology: Ecosystems and Human Activity
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of ecosystems, including the relationship between ecological balance and the sustainability of life;
- analyse natural and human threats to a local ecosystem and propose viable solutions to restore ecological balance;
- relate issues to environmental sustainability with a particular focus on issues in Ontario and Canada.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- describe the processes of photosynthesis and cellular respiration as they relate to the cycling of energy, carbon, and oxygen through abiotic and biotic components of an ecosystem (e.g., explain how glucose, water, and carbon dioxide are produced and/or consumed during these processes);
- illustrate the cycling of matter through biotic and abiotic components of an ecosystem by tracking nitrogen;
- illustrate the process of bioaccumulation through an example, and explain its potential impact on the viability and diversity of consumers at all trophic levels;
- show the relationship between the resources available and the equilibrium of a natural population in an ecosystem (e.g., describe the impact on an aquatic ecosystem of fishing or of harvesting a resource such as seaweed);
- explain why ecosystems with similar characteristics can exist in different geographical locations (e.g., why deserts exist in different parts of the world);
- describe how different ecosystems respond differently to short-term stresses and long-term changes (e.g., short term: the activity of tent caterpillars during a season; long-term: the effect of acid rain on maple trees);
- explain how soil composition and fertility can be altered in an ecosystem and outline the possible consequences of such changes.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- identify a current local concern or issue involving an ecosystem (e.g., the conversion of a grass lot into a parking lot; the impact of fishing on a lake; the building of a pulp and paper mill on a river; the construction of a hydroelectric dam);
- formulate scientific questions about the ecological issue and outline experimental procedures for finding answers;
- demonstrate the skills required to plan and conduct practical tests on related ecological factors, and collect data using appropriate instruments and techniques safely and accurately (e.g., tests for water quality, air quality, soil composition);
- select and integrate information from various sources, including electronic, print, and community resources, to answer the questions chosen;
- analyse the data and information gathered to clarify aspects of the concern or issue (e.g., identify costs and benefits from a social, cultural, and/or environmental perspective; predict the consequences of action or inaction; propose possible solutions);
- communicate the results of the investigation using a variety of oral, written, and graphic formats (e.g., write a letter to the mayor or organize a public debate);
- compile data on the biodiversity within a natural ecosystem, using appropriate techniques, and compare the results with those from a disturbed ecosystem.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- assess the impact of technological change on an ecosystem (e.g., the introduction of fertilizer and pesticides to soil; the introduction of a genetically engineered plant; the effect of polluted water or air on plants and animals);
- describe ways in which relationships between living organisms and their ecosystems are viewed by other cultures (e.g., First Nations);
- identify and evaluate Canadian initiatives in protecting Canada’s ecosystems;
- describe some of the technologies used in cleaning up contaminated sites;
- identify and describe careers based on ecology and environmental technology.
Chemistry: Chemical Reactions and Their Practical Applications
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of chemical reactions and the symbolic systems used to describe them;
- investigate chemical reactions encountered in everyday life and their practical applications;
- demonstrate an understanding of how chemical reactions relate to technological products and processes commonly encountered in everyday life.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- recognize the relationships among chemical formulae, composition, and names;
- demonstrate an understanding of chemical reactions, including conservation of mass, and their representation through balanced chemical equations;
- describe, using their observations, the reactants and products of a variety of chemical reactions, including synthesis, decomposition, and displacement reactions (e.g., the burning of magnesium, the production of oxygen from hydrogen peroxide, the reaction of iron in copper sulphate);
- describe qualitatively, using their observations, how factors such as heat, concentration, light, and surface area can affect rates of chemical reactions;
- classify substances as acids, bases, or salts based on their characteristic properties (e.g., reactions with indicators and with metals), names, and formulae (e.g., HCl, NaOH, NaCl);
- demonstrate an understanding of neutralization through investigation of simple acid-base reactions;
- describe how the pH scale is used to identify the concentration of acids and bases;
- name and write the formulae for common ionic and molecular
compounds (e.g., H
2 SO4 , NaNO3 , CO2 , NaOH).
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- select and use appropriate apparatus, and apply WHMIS safety procedures for the handling, storage, disposal, and recycling of laboratory materials (e.g., wear safety goggles and aprons; use proper techniques to handle, dispose of, and recycle acids, bases, and heavy metal ions; describe procedures to be followed in an emergency);
- formulate scientific questions about acid-base neutralization reactions and outline experimental procedures to answer the questions;
- demonstrate the skills required to plan and conduct practical experiments on acid-base neutralization reactions, and collect data using appropriate instruments and techniques in a safe and accurate manner (e.g., an experiment to neutralize a dilute solution of sodium hydroxide with dilute hydrochloric acid and extract the sodium chloride produced);
- select and integrate information from various sources, including electronic, print, and community resources, to answer the questions chosen;
- analyse the data and information gathered to clarify aspects of the questions chosen (e.g., data on changes in the acidity, fish populations, and clarity of Ontario’s small lakes over the years);
- communicate the results of the investigation, using a variety of oral, written, and graphic formats (e.g., use molecular models to represent chemical reactions);
- use the pH scale to determine the acidity or basicity of some common household substances (e.g., vinegar);
- conduct experiments to determine the factors that affect the rate of a chemical reaction (e.g., temperature, surface area of a solid, concentration of a solution);
- represent simple chemical reactions using word equations, balanced chemical equations, and, where appropriate, molecular models.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- use scientific nomenclature to identify common consumer products (e.g., identify ingredients in food products or cosmetics from the labels);
- investigate applications of acid-base reactions in common products and processes (e.g., compare the effectiveness of different brands of antacid tablets by quantitative analysis; prepare soap from lard and sodium hydroxide and compare its lather formation with that of commercial soaps);
- relate chemical reactions (including the rates of reactions) to familiar processes encountered in everyday life (e.g., acid-base reactions in film processing, food processing, fabric and hair dyeing, agriculture, wine making, pulp-and-paper and mineral processing) and identify careers that require knowledge of such processes (e.g., environmental engineering, swimming-pool maintenance);
- research the methods of chemical disposal used in Canada and the environmental and individual health and safety consequences of inappropriate disposal methods (e.g., examine the effects of dumping car batteries, tires, plastics, paints, or metals in landfill sites).
Earth and Space Science: Weather Systems
Overall Expectations
By the end of this course, students will:
- demonstrate an understanding of the factors affecting the fundamental processes of weather systems;
- investigate and analyse trends in local and global weather conditions in order to forecast local weather patterns;
- describe new technologies in meteorology and explain the impact of weather on our daily lives.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- identify and describe the principal characteristics of the hydrosphere and the four regions of the atmosphere;
- describe and explain heat transfer within the water cycle and how the hydrosphere and atmosphere act as heat sinks;
- describe and illustrate the factors affecting heat transfer within the water cycle in the atmosphere (e.g., temperature, pressure, humidity, winds);
- observe, through experiment and simulation, and describe (a) the effects of atmospheric pressure, (b) the pattern of air movement in convection, (c) the phenomenon of inversion, (d) the greenhouse effect, and (e) heat transfer through radiation (e.g., (a) the reduction of the boiling point of water with reduced presssure or altitude; (c) the formation of dew or frost early in the morning following a clear calm night; (e) the use of dark solar panels for effective heat transfer);
- describe the factors relating to the rotation of the Earth that cause the movement of air masses and variations in the Earth’s temperature;
- describe and explain heat transfer in the hydrosphere and atmosphere and its effects on air and water currents;
- describe and explain the effects of heat transfer within the hydrosphere and atmosphere on the development, severity, and movement of weather systems (e.g., effects such as pressure gradients, cloud formation, winds).
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- identify factors that affect the development, severity, and movement of local weather systems (e.g., microclimates in rural and urban areas, El Niño, bodies of water, frontal systems, smog);
- formulate scientific questions about these factors and outline experimental procedures for finding answers;
- demonstrate the skills required to plan and conduct a weather-related inquiry, and collect data using appropriate instruments and techniques safely and accurately (e.g., record temperatures and atmospheric pressure; interpret weather maps and satellite photographs);
- select and integrate information from various sources, including electronic, print, and community resources, to answer the questions chosen (e.g., historical trend data, local weather records, rates of evaporation of water);
- analyse the data and information gathered to clarify aspects of the questions chosen;
- communicate the results of the investigation, using a variety of oral, written, and graphic formats (e.g., diagrams, group presentations to the class, flow charts, simulations, graphs).
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- identify the impact of climate change on economic, social, and environmental conditions;
- describe examples of Canadian contributions to the field of meteorology (e.g., in satellite observation and imaging; in cold-climate meteorology);
- describe the impact of new technologies on our ability to predict local daily weather (e.g., Doppler radar, satellite imaging);
- assess the impact of weather on a variety of economic activities in Canada (e.g., agriculture, forestry, tourism, home construction, fruit growing).
Physics: Motion and Its Applications
Overall Expectations
By the end of this course, students will:
- describe different kinds of motion and the quantitative relationships among displacement, velocity, and acceleration;
- design and conduct investigations to study the displacement, velocity, and acceleration of a vehicle;
- identify ways in which the principles of motion are used in developing new technologies and describe the consequences of such developments.
Specific Expectations
Understanding Basic Concepts
By the end of this course, students will:
- distinguish among and provide examples of scalar and vector
quantities as they relate to the description of linear motion (e.g.,
among distance
 d,
displacement
d,
displacement 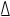
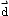 ,
and position
,
and position
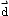 ,
and between speed v and velocity
,
and between speed v and velocity
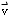 );
);
- distinguish among constant, instantaneous, and average speed and among constant, instantaneous, and average velocity, and give examples involving uniform and non-uniform motion;
- describe quantitatively the relationship among one-dimensional
average speed v
av , distance travelled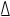 d, and
elapsed time
d, and
elapsed time 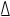 t,
and solve simple problems involving these physical quantities
t,
and solve simple problems involving these physical quantities
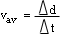 ;
;
- describe quantitatively the relationship among one-dimensional
average velocity
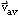 , displacement
, displacement
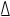
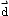 ,
and elapsed time
,
and elapsed time
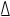 t, and solve
simple problems involving these physical quantities
t, and solve
simple problems involving these physical quantities
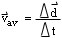 ;
;
- draw position-time graphs and calculate the average velocity and instantaneous velocity from such graphs;
- describe quantitatively the relationship among one-dimensional
average acceleration
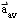 , change in
velocity
, change in
velocity 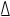
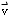 ,
and elapsed time
,
and elapsed time
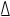 t, and solve
simple problems involving these physical quantities
t, and solve
simple problems involving these physical quantities
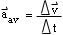 .
.
Developing Skills of Inquiry and Communication
By the end of this course, students will:
- through investigations and applications of basic concepts:
- formulate scientific questions about the motion of an object, including displacement, velocity, and acceleration, and outline experimental procedures for finding answers (e.g., “How can you accurately measure the displacement, velocity, and acceleration of a person, a bicycle, or a falling object?”);
- demonstrate the skills required to plan and conduct an inquiry into motion, identifying the variables to be measured, and collect data using appropriate instruments and techniques safely and accurately (e.g., measure and analyse an object’s motion in terms of displacement, velocity, and acceleration);
- select and integrate information from various sources, including electronic, print, and community resources, to answer the questions chosen (e.g., compare the characteristics of the different object motions investigated);
- analyse the data and information gathered to clarify aspects of the chosen questions (e.g., estimate journey times from road maps and average speeds);
- communicate the results of the investigation using a variety of oral, written, and graphic formats.
Relating Science to Technology, Society, and the Environment
By the end of this course, students will:
- perform a cost-benefit analysis, including environmental and safety factors, of technologies which have enabled us to attain ever-faster speeds on land and water and in the air, and of alternative modes of transportation (e.g., snowmobiles, automobiles, trains, subways);
- investigate the benefits and risks to the community and the individual of alternatives to motor-vehicle transportation (e.g., public transit, high-speed trains, walking, bicycling, in-line skating, horseback riding, skiing);
- describe examples of Canadian and other contributions to the science and technology of motion (e.g., snow vehicles, aircraft, hydrofoils, the G-suit, the canoe, the spring skate).
|
|
|
Some Considerations for Program Planning in Science |
Teachers who are planning a program in science must take into account considerations in a number of important areas. Essential information that pertains to all disciplines is provided in the companion piece to this document, The Ontario Curriculum, Grades 9 to 12: Program Planning and Assessment, 2000. The areas of concern to all teachers that are outlined there include the following:
- types of secondary school courses
- education for exceptional students
- the role of technology in the curriculum
- English as a second language (ESL) and English literacy development (ELD)
- career education
- cooperative education and other workplace experiences
- health and safety
Considerations relating to the areas listed above that have particular relevance for program planning in science are noted here.
Education for Exceptional Students. In planning programs in science, teachers should recognize that exceptional students may require focused and specialized directions, as well as advance instruction and additional practice in the use of equipment. Issues relating to safety in the laboratory and to students’ ability to read laboratory manuals and use laboratory equipment must be addressed before students can be expected to participate effectively. Changes to teaching materials may involve the use of large-print activity sheets, the highlighting of key points on print materials, and the use of alternative texts at a suitable reading level. Assessment strategies should allow students to demonstrate their understanding of scientific concepts in a variety of ways, such as by performing experiments, creating displays and models, and tape-recording observations. Computer programs may be used to provide opportunities for scientific practice and for recording results.
The Role of Technology in the Curriculum. In science, students gain “hands-on” experience with technology in the laboratory. Apparatus as diverse as digital balances and volumetric apparatus in chemistry, microscopes and Petri dishes in biology, and air tables and ammeters in physics provide the kinesthetic learner with unique learning experiences. Computers can be used in science to support laboratory investigations; for example, electronic probes can be used to monitor variables such as temperature, pH, and velocity. Computer programs can also be used to process class data and to simulate environmental or industrial scenarios, or animal dissections. Care must be taken, however, to ensure that computer-assisted laboratory programs are not used in situations where students’ own technical skills should be developed, such as in analysing and graphing data.
The Internet is a particularly valuable source of scientific information that students should be taught to access. In addition, some programs enable students to conduct scientific investigations and then use the tools of electronic communication to compare their results and analyses with those of students in other parts of Canada and around the world.
English As a Second Language and English Literacy Development (ESL/ELD). Science presents particular linguistic challenges to all students because of its specialized terminology and language structures. Science teachers who have ESL/ELD students in their classes should respond to the special needs of these students, providing support with respect to their comprehension and use of language in a scientific context.
Career Education. Ongoing scientific discoveries, coupled with rapidly evolving technologies, have resulted in an exciting environment in which creativity and innovation thrive, bringing about new career opportunities. Today’s employers seek candidates with strong critical-thinking and problem-solving skills and the ability to work cooperatively in a team environment – traits that are developed through participation in the science program. The program should be designed to give students an opportunity to explore science-related careers.
Cooperative Education and Other Workplace Experiences. Through participation in science-related learning activities in commercial, industrial, government, or academic laboratory settings, students can experience the application of knowledge and skills in specific areas of science in settings outside the school. These experiences give students the opportunity to practise and develop their own skills in problem solving, critical thinking, teamwork, and the safe and accurate use of scientific procedures and tools. In addition, they provide students with a clearer sense of the nature of careers in science-related fields.
Health and Safety. Teachers are responsible for ensuring the safety of students during classroom activities and for teaching students to assume responsibility for their own and others’ safety. They must model safe practices and communicate safety expectations to students in accordance with school board and ministry policies.This concern for safety in science requires that students demonstrate:
- knowledge about the materials, tools, processes, and procedures used in science;
- skill in performing tasks in the laboratory;
- knowledge about health and safety concerns and about the care of living things (plants and animals) that are brought into the classroom;
- concern for the health and safety of self and others.
Students demonstrate the knowledge, skills, and habits of mind required for safe involvement in science when they, for example:
- maintain a well-organized and uncluttered work space;
- carefully follow the instructions and example of the teacher;
- identify possible health and safety concerns;
- follow established safety procedures;
- suggest and implement appropriate safety procedures in new situations;
- comply with Workplace Hazardous Materials Information System (WHMIS) legislation.
|
|
|
The Achievement Chartfor Science |
The achievement chart that follows identifies four categories of knowledge and skills in science – Knowledge/Understanding, Inquiry, Communication, and Making Connections. These categories encompass all the curriculum expectations in courses in the discipline. For each of the category statements in the left-hand column, the levels of student achievement are described. (Detailed information on the achievement levels and on assessment, evaluation, and reporting policy is provided in The Ontario Curriculum, Grades 9 to 12: Program Planning and Assessment, 2000.)
The achievement chart is meant to guide teachers in:
- planning instruction and learning activities that will lead to the achievement of the curriculum expectations in a course;
- planning assessment strategies that will accurately assess students’ achievement of the curriculum expectations;
- selecting samples of student work that provide evidence of achievement at particular levels;
- providing descriptive feedback to students on their current achievement and suggesting strategies for improvement;
- determining, towards the end of a course, the student’s most consistent level of achievement of the curriculum expectations as reflected in his or her course work;
- devising a method of final evaluation;
- assigning a final grade.
The achievement chart can guide students in:
- assessing their own learning;
- planning strategies for improvement, with the help of their teachers.
The achievement chart provides a standard province-wide method for teachers to use in assessing and evaluating their students’ achievement. Teachers will be provided with materials that will assist them in improving their assessment methods and strategies and, hence, their assessment of student achievement. These materials will contain samples of student work (exemplars) that illustrate achievement at each of the levels (represented by associated percentage grade ranges). Until these materials are provided, teachers may continue to follow their current assessment and evaluation practices.
To ensure consistency in assessment and reporting across the province, the ministry will provide samples of student work that reflect achievement based on the provincial standard, and other resources based on the achievement charts. As these resources become available, teachers will begin to use the achievement charts in their assessment and evaluation practices.
To support this process, the ministry will provide the following:
- a standard provincial report card, with an accompanying guide
- course profiles
- exemplars
- curriculum and assessment videos
- training materials
- an electronic curriculum planner
When planning courses and assessment, teachers should review the required curriculum expectations and link them to the categories to which they relate. They should ensure that all the expectations are accounted for in instruction, and that achievement of expectations is assessed within the appropriate categories. The descriptions of the levels of achievement given in the chart should be used to identify the level at which the student has achieved the expectations. Students should be given numerous and varied opportunities to demonstrate their achievement of the expectations across the four categories. Teachers may find it useful to provide students with examples of work at the different levels of achievement.